All I got was a 41 band - do I have Lyme? Lyme test is IgM positive, could it be chronic Lyme?
Its complicated.
If the text is too hard to follow or boring, there are pictures below.
It helps if you know a little about laboratory medicine.
The Western Blot and ELISA tests, the tests most often used, are indirect tests. Rather than looking for direct evidence of infection, e.g. finding specific DNA, These tests look for foot prints: Antibodies or specific immunoglobulins. These tiny proteins are made by the immune system in response to recognized pathogens (disease-causing) germs: bacteria, fungi, protozoans and viruses primarily.
There are a lot of reasons why Lyme antibody tests are not that great: People are genetically programmed to respond differently; Lyme strains vary, species vary; the commonly used test is based on a single Lyme strain, you may be infected by another strain and cross reactivity with the test may be minimal. Perhaps most important: the test is incorrectly calibrated, based on false assertions. This is my opinion and is shared by others.
Before we get to the Western Blot, let's first discuss the ELISA. Enzyme Linked Immunosorbent assay. This is the first test, the first tier used in the two tier test recommended by the CDC and IDSA.
All diagnostic serological (from blood-serum) lab tests need controls, positive and negative.
A positive control is a uses a standard serum with a high level of antibodies directed against Borrelia burgdorferi B31 strain. A negative control has no Lyme antibodies and is ostensibly derived from serum with no Lyme antibodies. (The availability of true negative controls has been a debatable point). The patient serum, which may or may not have Lyme antibodies, is tested with B31 derived antigen.
The test requires a supply of Lyme antigen -- proteins and polysaccharides, which stimulate antibody formation derived from the B31 stain of Lyme. Antigens are sourced from a culture of Lyme bacteria. The culture may be "high passage." This means that the original culture has been "replanted" over many generations. Mutations may occur and change the antigens overtime.
The test requires a supply of Lyme antigen -- proteins and polysaccharides, which stimulate antibody formation derived from the B31 stain of Lyme. Antigens are sourced from a culture of Lyme bacteria. The culture may be "high passage." This means that the original culture has been "replanted" over many generations. Mutations may occur and change the antigens overtime.
A culture of Lyme bacteria is put in a "blender," or sonicated to create a homogenate.
ELISA. Sticky Lyme antigen is adhered to the surface of small wells . Serum is added. Lyme antibodies present in the patient test serum should tightly bind to the Lyme antigens on the bottom of the well. There is some nonspecific binding by "promiscuous" antibodies that apparently will stick to anything. The test well are washed thoroughly to remove debris and nonspecific antibodies. Sera is added in three separate experiments: positive control, negative control and patient serum. If the patient has Lyme antibodies the patient test wells should closely resemble the positive control.
ELISA stands for Enzyme linked Immuno-sorbent assay. Antibodies are shaped like a Y with three legs and 3 points of contact which may bind to a receptor In the experiment described above, a secondary antibody is added to the well and this antibody specifically binds to a free receptor on Lyme antibodies bound to antigen on the surface of the wells. A protein called an enzyme is attached to a leg of this "secondary antibody". A color change occurs when a reagent is added, reacting with the bound enzyme. The intensity of color increases if more antibodies are bound to the antigen. Patient serum with a high concentration of Lyme antibodies and the positive control induce an intense color change.. If no Lyme antibodies are present no color change or only a slight color change occurs. A mild amount of color is thought to be normal and due to nonspecific, promiscuous antibodies bound to the Lyme antigens. Basically: More color, more antibodies; less color, fewer antibodies. A machine called a colorimeter measures the color intensity and the results are recorded as an index. A positive index is set based on "standard" recommendations.
This test has the advantage of including all antibodies that might be present in the patient's serum. Antigens may be polysaccharide or protein. The Western Blot only measures responses to protein antigens.
ELISA tests may have false negatives and false positives.
The positive control is based on an ideal serum with a robust immune response, typically found in the acute phase of the disease. Excluded are: patients who have been sick for a long time, patients who have a weak immune response, patients with a different strain of Lyme and many others.
This test has the advantage of including all antibodies that might be present in the patient's serum. Antigens may be polysaccharide or protein. The Western Blot only measures responses to protein antigens.
ELISA tests may have false negatives and false positives.
The positive control is based on an ideal serum with a robust immune response, typically found in the acute phase of the disease. Excluded are: patients who have been sick for a long time, patients who have a weak immune response, patients with a different strain of Lyme and many others.
The test is predicated on a single strain of Borrelia burgdorferi, the B31 strain. The test will like fail when a patient is infected with other strains and/or species of Lyme. This phenomenon is well known and well described in peer reviewed literature.
Doctors talk about whether or not a test is sensitive or it is specific.
A test is sensitive if it excludes false negatives. A test is specific when all the positive are "true positives." There is always a balance. When you increase sensitivity you decrease specificity. When you increase specificity you decrease sensitivity.
Doctors talk about whether or not a test is sensitive or it is specific.
A test is sensitive if it excludes false negatives. A test is specific when all the positive are "true positives." There is always a balance. When you increase sensitivity you decrease specificity. When you increase specificity you decrease sensitivity.
/Lyme testing immediately raises a red flag. Why are two steps required, an ELISA followed by a Western Blot? The only other 2 tier test was for HIV. With improved technology a Western blot is no longer required with HIV testing. The war over Lyme testing is part of the war over most things Lyme.
The Western Blot is considered both a sensitive and specific test. It is superior to the ELISA. A mix of Lyme antigens is placed into a gel. When an electric current is applied, Lyme proteins, antigens, separate out based on the weight of individual proteins. Antigenic proteins are spread out in a linear fashion across a gel. The separated proteins are transferred to a strip of nitrocellulose -- something like a strip of paper. The test proteins typically have molecular weights of 18 kilo Daltons to 93 kilo Daltons.
The antigen/protein laden strips are incubated with serum. Three strips should be tested: positive control, negative control and patient serum. Antibodies present in the serum will bind to the individual proteins on the strips. A reagent is added and bars or bands appear across the strip.
Band position and name corresponds to the molecular weight of the particular Lyme antigen.
Some bands are very specific. The likelihood of non specific antibody binding to the specific protein is very low. The CDC formula is based on a random number of bands and does consider the importance of particular bands. The CDC standard is derived from a 1994 convention at which time a standard for surveillance was establishedBand position and name corresponds to the molecular weight of the particular Lyme antigen.
The WB shares with the ELISA the problem of what constitutes a positive reaction.
A picture is worth a thousand words. Let me a review a few images, typical of what I work with on a daily basis.
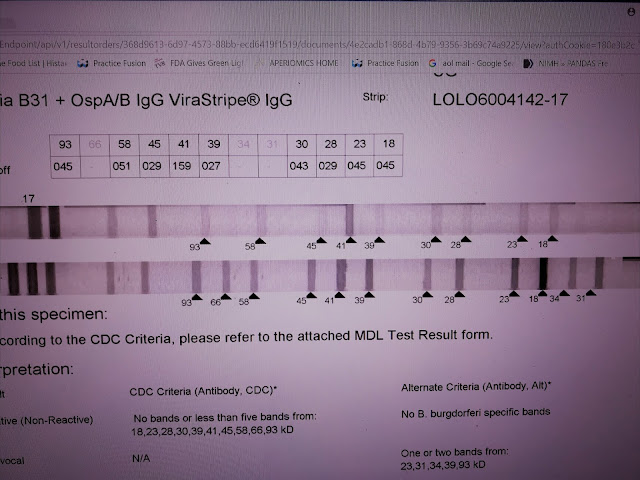
These results are from MDL. A specialty laboratory in NJ. This is an IgG strip. The test measures responses to 10 standard antigens and to the specific 31 and 34 bands removed decades ago because of vaccine development. The Bands are separated based on weight, 18-93. The 31 and 34 are added with a separate test so the numbers are out of place. The bottom strip is the positive control - high levels of specific antibody directed against the blot proteins. The top line is the patient result. No negative control is provided.
CAVEAT; INTERPRETATIONS DISCUSSED ARE THE OPINION OF THE AUTHOR AND MAY BE CONTROVERSIAL AND OPPOSED BY MOST AUTHORITIES.
This test is CDC negative. Patient antibodies react to nearly every band. This strip appears blatantly positive. However, according to the manufacturer, the intensity of the response is too weak to call positive. A band is considered positive if the intensity is at least 60% of the control. For example, the 93 band has an intensity of 45% of the control. Based on this standard, only the 41 band is counted positive. This interpretation is wrong, I think, because the lower limit of a positive results is incorrect -- based on incorrect assumptions regarding the control. I call this test positive. The total number of reactions is important and bands with intensities of 30% or higher should be considered.
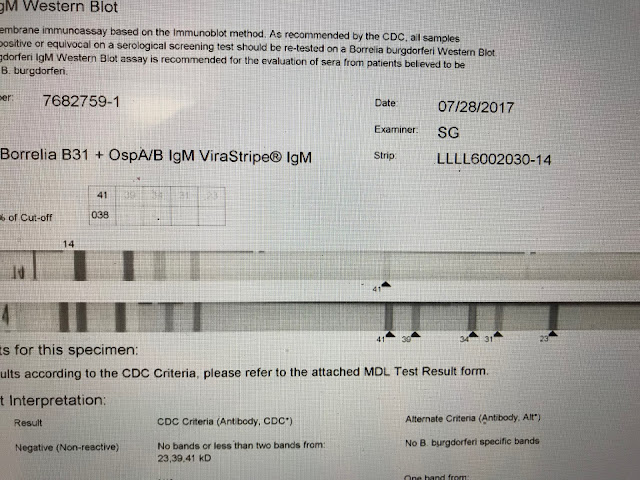
This is a negative IgM strip. Only the 41 band appears. The diagnosis of Lyme should not be based solely on the appearance of the 41 band which itself is considered less specific than several others.
This is another example of a CDC negative which I consider positive. The 4 reported bands are all specific. The 39 band -- just 4 percentage points shy of the lab internal cutoff, is perhaps the most specific band, and to the best of my knowledge cross reactivity does not exist. The test is positive by other non-CDC internal laboratory criteria.
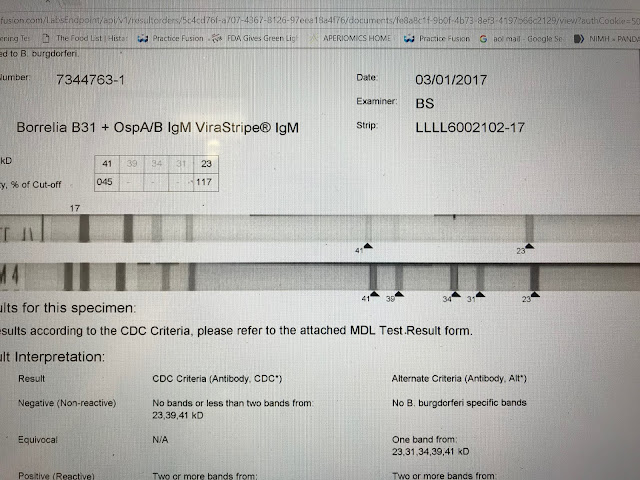
This is a positive IgM strip. The test is reported CDC negative. The 41 and 23 bands together are considered positive by the CDC standard. There is a general consensus this criteria is accurate. The test would be reported negative however because the 41 intensity falls below the cut off point of 60%. What is of relevance, not taken into account by automated reading, is that the 23 band, the more specific band, is of an intensity greater than the control and therefore of greater significance.
This is a typical LabCorp or Quest report. As you see, bands are not represented numerically. Bands are reported present or absent. The results are not determined by a computer which measures pixels, as in the case of MDL, but rather by a technician eyeballing the strips. These bands are highly specific, especially the 39 band. I consider this strip likely positive but I would repeat the test from a reference lab before passing judgement.
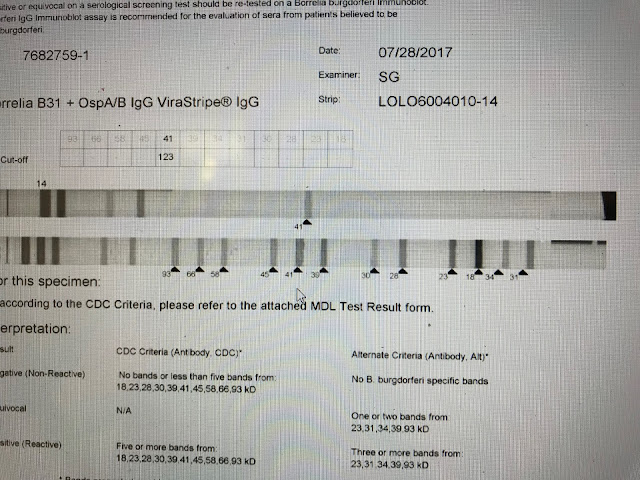
This is a negative. On
this IgG strip only the 41 band is apparent and this is not enough to confirm
Lyme serologically.
This is report from Stony Brook. CDC bands are called specific and non-CDC bands are called nonspecific. Novel bands, not available through other reference labs are reported and may be very helpful. Unfortunately , no images.
This Blot is positive although it is CDC negative. The CDC requires 5 specific bands not present on this strip. Several very specific bands are clearly present.
This report is from IgeneX. Results are presented is a different format.
IgM: is it chronic Lyme?
I don't care if the bands are IgM or IgG. Lyme IgM bands may persist for years after acute infection. IgG bands may never appear.
The 41 band as the sole finding does not lead credence to the diagnosis of Lyme. It also does not exclude the diagnosis.
Lyme antibodies do not fit into the traditional mold. For example, IgM antibodies to the 31 and 34 bands may be present, even though these antigenic proteins do not appear until at least 6 months following initial infection. The 31 band is associated with outer surface protein A. This protein is expressed with attachment to the tick gut and down-regulates - disappears after infection. It only shows up in the host many months after acute infection. If you believe that Lyme IgM antibodies disappear within weeks after acute infection, as proposed by the CDC, this paradox is impossible to explain.
There is a story of repeated by physicians which confuses patients as well as their doctors. This is how the immune system works: IgM and IgG responses are predictable. IgM antibodies appear early, immediately after infection. The better, protective IgG antibodies quickly emerge as IgM antibodies decline. When the patient recovers from the illness, higher levels of IgG antibodies are present and no IgM is detectable. Therefore in late Lyme, only IgG antibodies are present.
Nice story, but wrong in the case of Lyme. Other infections may have unexpected antibody responses.
How do I interpret results? First, it is important to recognize limitations of these tests. The tests are not automated, many steps performed by humans. Therefore, the chance of error is high. Generally, the results from the 3 reference labs are trustworthy. Sometimes repeating the test is helpful. I do not follow any particular guideline approach. Standards which require 2 of the following or 5 etc. lock you in. I would rather interpret results case by case. In my opinion, reading a Western Blot is something like reading an X-ray. A lot of factors are considered -- on the other hand, sometimes Interpreting Blots is like reading tea leaves. Lab tests are adjunctive and cannot be relied upon as the sole means of diagnosing this complex, enigmatic disorder called Lyme disease.
Even more important: the CDC standard is for surveillance -- for purposes of monitoring the relative number of cases in different locations over time. This should not be relied upon as a diagnostic standard.





5 comments:
Thanks for sharing such wonderful information about Best Allergy Test In India
So an indeterminate western Blot Lyme Igm lab result is *31 and *41 only
IGeneX says to retest if only these numbers come up on test. Would this mean that with those 2bands he is positive for Lyme?
Son tested Babesia duncani FISH. Positive
Babesia duncani IgM <20
Babesia duncani IgG 160 Positive
Thanks for your blog, very informative
descargar apk
descargar root explorer apk
descargar whatsapp plus apk
Thank your very much for informarion and opinion, so helpfull for me. What do you think about testing after antibiotics, is it true,that after them, the antibodies amount is bigger due o reaction on death borrelia,or not? Do you see more real,that borrelia gonin persister form and so after antibiotics,the amount if antibiotics is lower?
Amount of antibodies I meant🙂
Post a Comment